10. Ethylene Glycol
It’s highly likely that you have a bottle of this first chemical lying around somewhere in your garage. Ethylene glycol, the main ingredient in anti-freeze, is a common household chemical used as a coolant in cars. However, it’s also a dangerous poison.
In the body, it’s converted into glycolaldehyde by the same enzyme that breaks down the alcohol you’d find in beer or wine. Once this occurs, the glycolaldehyde is oxidised into a substance called glycolic acid, which is about as nasty as it sounds. The acid disrupts the body’s delicate pH balance and also has a cytotoxic effect, meaning that it kills cells.
The kidneys and central nervous system are the primary systems that are damaged by the antifreeze chemical. Ethylene glycol didn’t make this list just for its poisonous effects, however. The dangerous chemical also has a notoriously sweet taste, meaning that children, pets and even unwitting adults have been known to guzzle it by mistake and then suffer its negative effects. Talk about a sweet-faced killer!
First Identified: 1856 CE
Chemical Formula: C2H6O2
Where You Might Find it: Computer and automobile coolants, antifreeze, some air conditioning systems
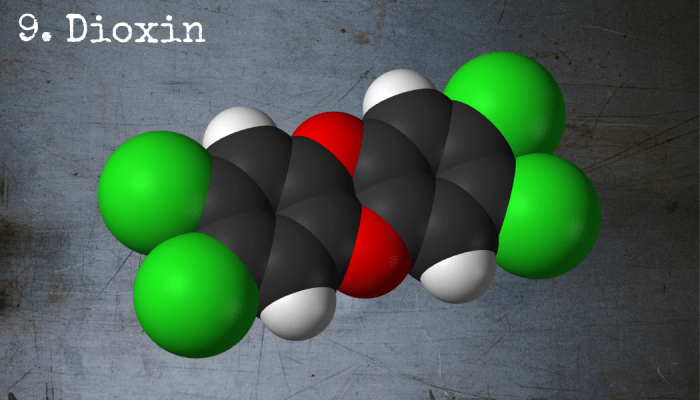
Dioxin is a highly toxic, fat-soluble chemical that causes damage to organs and lesions on the skin.
Annie Spratt via Unsplash; Public Domain via Wikimedia Commons; Canva
9. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin
Aside from having a killer name, 2,3,7,8-tetrachlorodibenzo-p-dioxin—often referred to as TCDD or simply dioxin—is a highly toxic compound that can be produced as a byproduct of incomplete combustion (i.e. combustion without enough oxygen present). The chemical causes lesions on the body known as chloracne and damages fatty organs like the liver, spleen and intestines.
This is because dioxin is a fat-soluble molecule and thus has a nasty tendency to accumulate in the body’s fatty tissues and then stick around. One of the scariest things about this chemical is that we don’t really know how it works or why it has such severe effects, which means that treatment for dioxin poisoning is a bit of a guessing game.
First Identified: 1897 CE
Chemical Formula: C12H4Cl4O2
Where You Might Find it: The fat of meat, fish and dairy contaminated via industrial processes
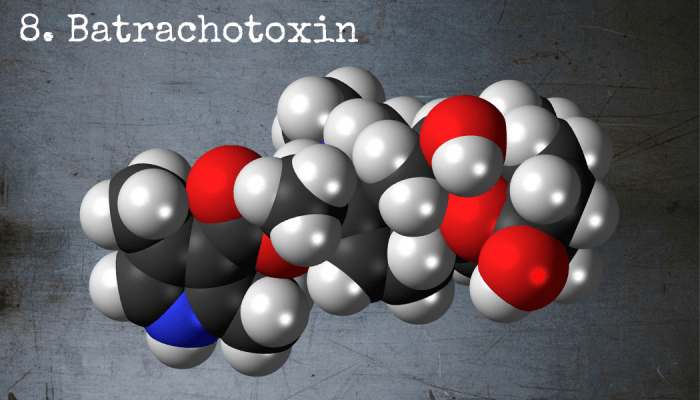
Batrachotoxin exists naturally on the skin of certain poisonous frogs native to South America.
Annie Spratt via Unsplash; WikimediaImages via Pixabay; Canva
8. Batrachotoxin
Batrachotoxin, which is found on the skin of certain frogs native to South America, is one of the most potent poisons known to man. It takes just two micrograms per kilogram to be fatal, which means that a fully grown man could be killed by a dose no bigger than a few grains of salt. It’s a neurotoxin, which means that it exerts its effect by preventing neurones from sending electrical messages to one another, causing paralysis and eventually death. Scary stuff!
First Identified: 1960s CE
Chemical Formula: C31H42N2O6
Where You Might Find it: The skin of poison dart frogs
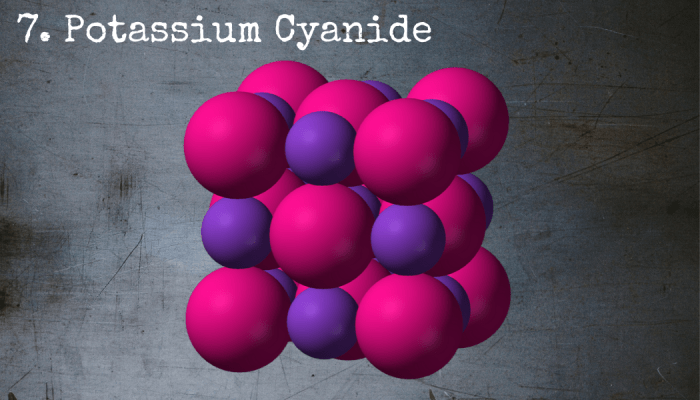
Potassium cyanide is said to be the active ingredient in the suicide pills reportedly carried by spies and special ops soldiers.
Annie Spratt via Unsplash; Ben Mills, Public Domain via Wikimedia Commons; Canva
7. Potassium Cyanide
Potassium cyanide is a salt, but it’s about as far from the kind that you'd use to season your fries as possible. It’s incredibly toxic and has gained notoriety for being the choice ingredient in suicide pills for spies and soldiers around the world. The nicest thing that can be said about it is that it offers a quick death.
It disables cellular respiration, the process by which cells make energy, by inhibiting an enzyme that’s essential in ATP production. ATP is the primary energy currency of the body, and the ability to make it is key to, well, living. Within a few minutes of consuming potassium cyanide, victims fall unconscious and then suffer brain death. Yikes!
First Identified: 1752 CE
Chemical Formula: KCN
Where You Might Find it: Ore-processing facilities, some photographic fixers
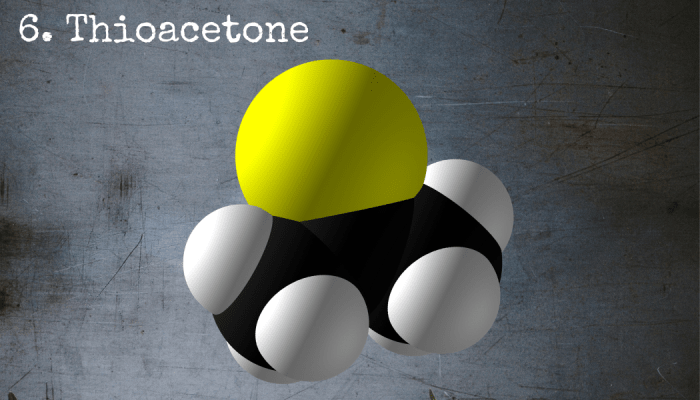
Thioacetone just might be the worst-smelling chemical on earth.
Annie Spratt via unsplash; PishT, C-BY-SA-4.0 via Wikimedia Commons; Canva
6. Thioacetone
Thioacetone isn’t poisonous. It isn’t corrosive, explosive or even particularly volatile. However, it has one special property that makes it one of the most dangerous chemicals on earth: its smell. The stench of thioacetone has been described as "fearful," and it causes anyone in its vicinity to vomit, faint or flee with horror.
To get an understanding of just how terrible this smell is, a story is warranted. In 1889, a group of scientists in the German town of Freiburg were working on a related compound and accidentally managed to synthesise some thioacetone. The stench could be detected from half a kilometre away, and it triggered an evacuation of the entire town as people began vomiting uncontrollably. In summation, thioacetone won’t kill you, but it’ll probably make you wish you were dead.
First Identified: 1889 CE
Chemical Formula: C3H6S
Where You Might Find it: Some chemistry labs
Before the sickness and death of Karen Wetterhahn in 1996, the extent of the danger posed by dimethylmercury was not widely known.
Annie Spratt via Unsplash; Public Domain via Wikimedia Commons; Canva
5. Dimethylmercury
Dimethylmercury is a simple little molecule consisting of a central mercury atom bonded to two methyl (CH3) groups. The toxic effects of mercury are known to pretty much everyone, but few are aware that the liquid metal on its own is actually fairly harmless. It can’t bind to any tissues in the body on its own and therefore can’t be absorbed. However, the addition of the two methyl groups in dimethylmercury means that the compound can be readily absorbed into the blood and transported all around the body where it can exert its toxic effect.
The true dangers of working with dimethylmercury came to light in 1996, when chemist Karen Wetterhahn accidentally spilled two drops of the chemical on her glove while working in the lab. Assuming that the latex would prevent the chemical from coming into contact with her skin, she didn’t fret. After a few months, however, she began to exhibit signs of cognitive impairment. Slurred speech, difficulty thinking and fatigue soon gave way to a coma. After five months, her coma finally ended in death.
First Identified: 1858 CE
Chemical Formula: HgC2H6
Where You Might Find it: Reference toxin sets
Fluoroantimonic acid is so corrosive that it cannot be stored or studied in the glass containers typically used by chemists.
4. Fluoroantimonic Acid
Fluoroantimonic acid is the strongest acid in the world. Ever head of sulphuric acid? Well, it’s about ten quadrillion times stronger than that. The compound can eat through plastic and glass and could melt the skin from your bones and still be hungry for more.
The only way that it can be
stored is in Teflon containers, which are resistant to its corrosive
effects. When studying it, scientists aren’t even able to use normal
glass beakers unless they dilute it by a factor of thousands. What’s
more, fluoroantimonic acid also reacts violently with water. Fun stuff!
First Identified: Not available
Chemical Formula: H2SbF6
Where You Might Find it: Tetraxenon gold compound manufacturing facilities
Largely due to the high-energy arrangement of its constituent atoms, azidoazide azide is an extremely volatile explosive.
3. Azidoazide Azide
1-diazidocarbamoyl-5-azidotetrazole, or azidoazide azide, is the most volatile explosive compound currently known to man. It’s comprised of 14 nitrogen atoms loosely bound in a high-energy conformation. When a molecule is in a high-energy conformation, it seeks to move down to a lower energy state, and when this transition occurs, energy is released.
Azidoazide azide is an extreme case of this phenomenon, in which its high energy conformation is so unstable that pretty much anything can make it explode. The slightest pressure or friction, small temperature fluctuations or even exposure to light can cause it to go boom. In fact, it’s so volatile that the normal instruments used to measure how unstable a substance is can’t be used on it. In other words, it’s too explosive to measure how explosive it is. Eeek!
First Identified: Not available
Chemical Formula: C2N14
Where You Might Find it: Almost nowhere, possibly some chemistry labs
Chlorine trifluoride, first discovered by the Nazis during WWII, is corrosive enough to eat through concrete.
Annie Spratt via Unsplash; Public Domain via Wikimedia Commons; Canva
2. Chlorine Trifluoride
Chlorine trifluoride, also known as substance N, was discovered by Nazi scientists during the Second World War. The Nazi Party initially intended to have their soldiers use it to melt through Allied bunkers, but after years of research, they determined that it was just too unstable. That’s right, this chemical was too destructive for the Nazis. It’s extremely volatile and will react explosively with just about anything. It’s been known to set fire to glass, sand, rust and, of course, people. It can even cause asbestos—one of the most fire-retardant substances in existence—to catch fire.
The United States briefly tinkered with chlorine trifluoride and attempted to transport a tonne of it in a specialised tanker. This turned out to be a really, really bad move. The tanker crashed and the substance spilled out onto the concrete floor of a warehouse and set fire to it. It ate through the concrete completely along with a good few feet of the dirt and gravel beneath. I don’t know about you, but I don’t want this stuff within a thousand miles of me.
First Identified: 1930s CE
Chemical Formula: ClF3
Where You Might Find it: Rocket propellant, semiconductor cleaner
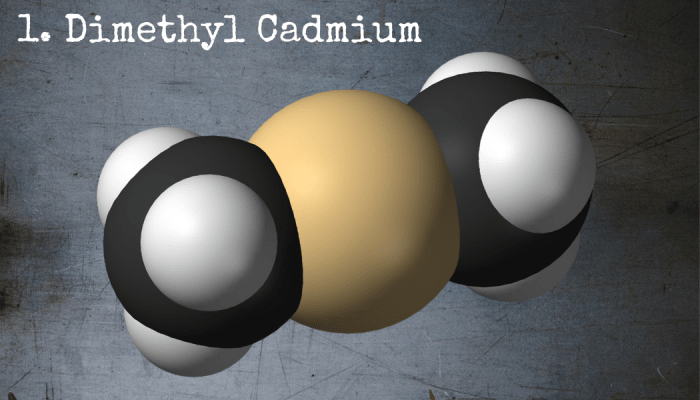
This innocent-looking little molecule, dimethyl cadmium, is arguably the most dangerous chemical in the world.
Annie Spratt via Unsplash; Public Domain via Wikimedia Commons; Canva
1. Dimethylcadmium: The Deadliest Chemical in the World?
Even worse than dimethylmercury, dimethylcadmium is considered by many chemists to be the most toxic chemical known to man. Because cadmium is lighter than mercury, the organic compound is more volatile. It absorbs instantly into the bloodstream and rips apart the organs that need the highest supply of blood, including two little body parts that you might have heard of called the heart and the lungs.
If, by some miracle, a person manages to survive the initial exposure, the danger certainly isn’t over. Dimethylcadmium is highly carcinogenic, meaning that it causes cancer. If that isn’t bad enough, it also explodes in water and decomposes into dimethyl calcium peroxide, which is highly explosive. In summation, it’s a volatile, poisonous, cancer-causing, explosive and vicious little molecule that can easily be called the most dangerous chemical known to man. It’s no wonder, really, that the majority of the world’s chemists refuse to work with it.
First Identified: Not available
Chemical Formula: C2H6Cd
Where You Might Find it: Formerly in labs
What to Do If You Are Exposed to a Toxic Chemical
If for any reason you ingest or come into contact with a potentially harmful chemical substance, call 911 (or the emergency services number for your country/state) immediately and request help from medical authorities. For additional help, contact the 24-hour American Association of Poison Control Centers at 1-800-222-1222. If a pet or animal has been exposed, contact the ASPCA National Animal Poison Control Center at 1-888-426-4435.
Because of its sweet taste, antifreeze, which contains ethylene glycol, has caused unintended deaths and injuries in pets and humans alike.
Annie Spratt via Unsplash; Ben Mills, Public Domain via Wikimedia Commons; Canva
Comments
Post a Comment